Early Safety Assessment - Drug Discovery and Development Based on
Por um escritor misterioso
Last updated 01 julho 2024

The drug candidate faces numerous efficacy and safety hurdles before moving forward to clinical testing. Here at the UPDDI we recognize the need for early identification of potential human toxicity and pharmacokinetic issues by creating a unique human liver microphysiological systems platform for drug testing before implementing preclinical animal testing.
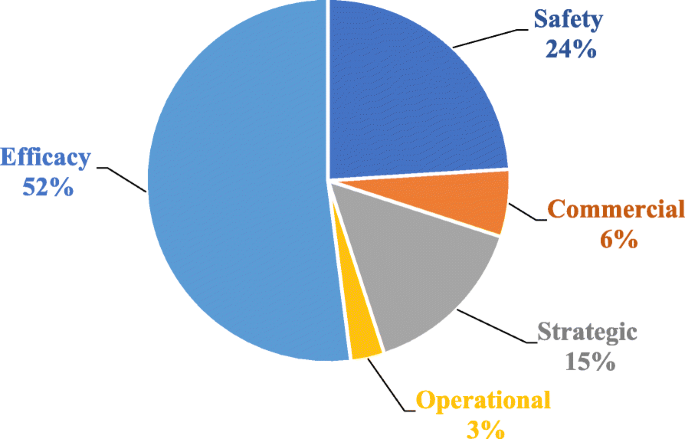
Exploring different approaches to improve the success of drug

Moving Preclinical Safety Evaluation from Hazard Identification

Phases of Drug Development Process, Drug Discovery Process
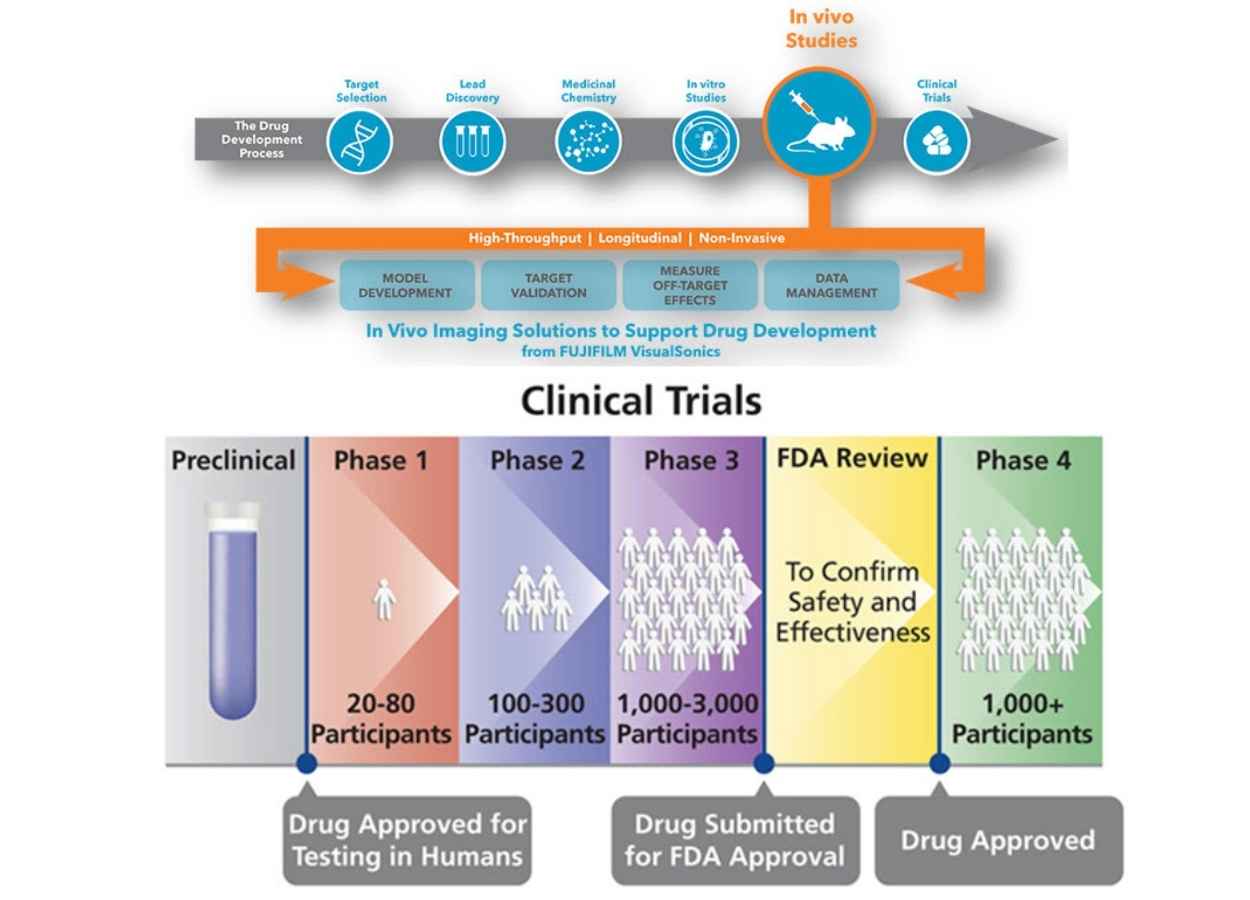
AN OVERVIEW OF NEW DRUG DISCOVERY AND DEVELOPMENT

Biomarkers in efficacy and safety assessments. Application of in
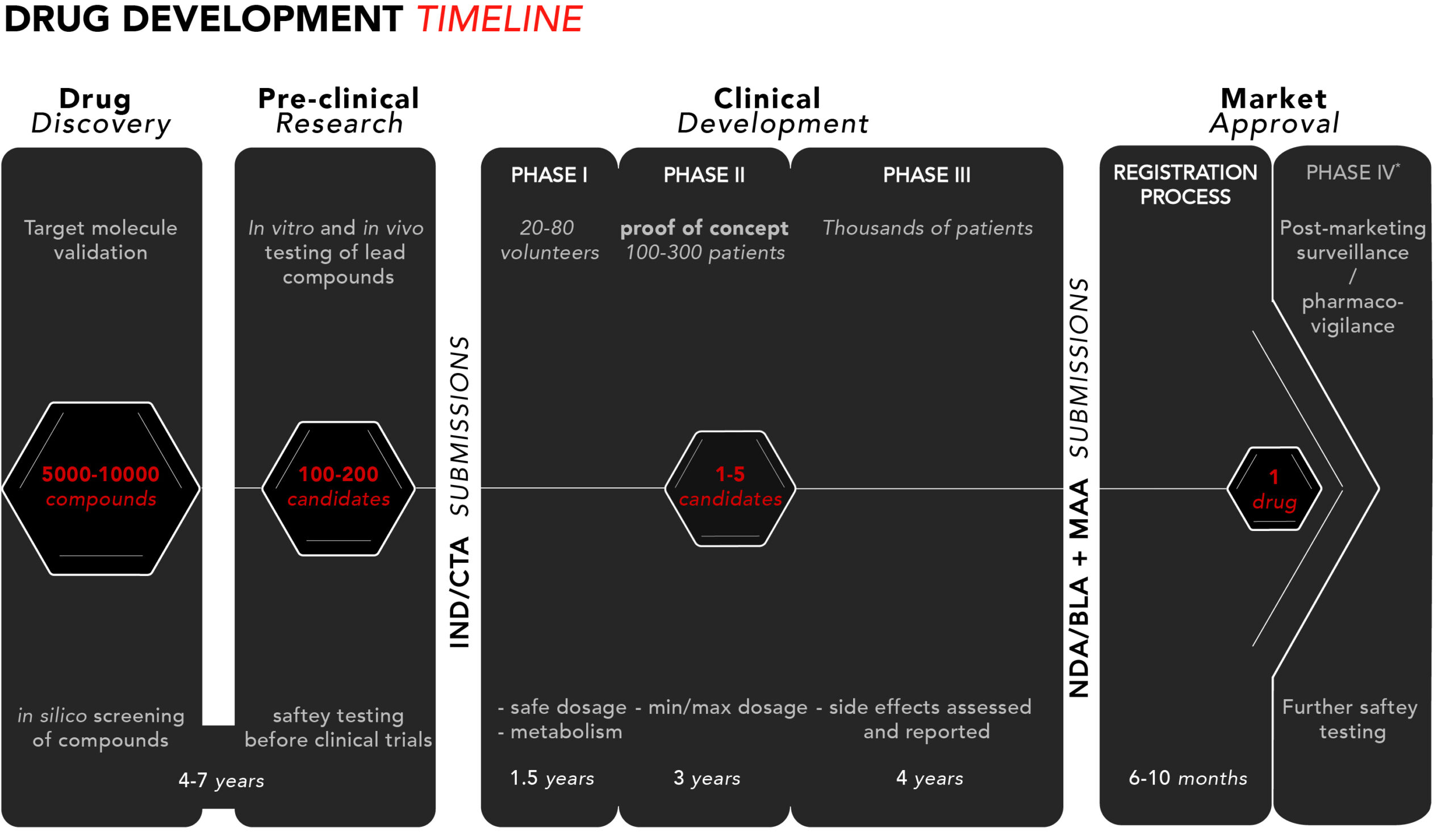
Drug development – The four phases - BioStock

Principles of early drug discovery - Hughes - 2011 - British

PDF] In silico Toxicology-A Tool for Early Safety Evaluation of
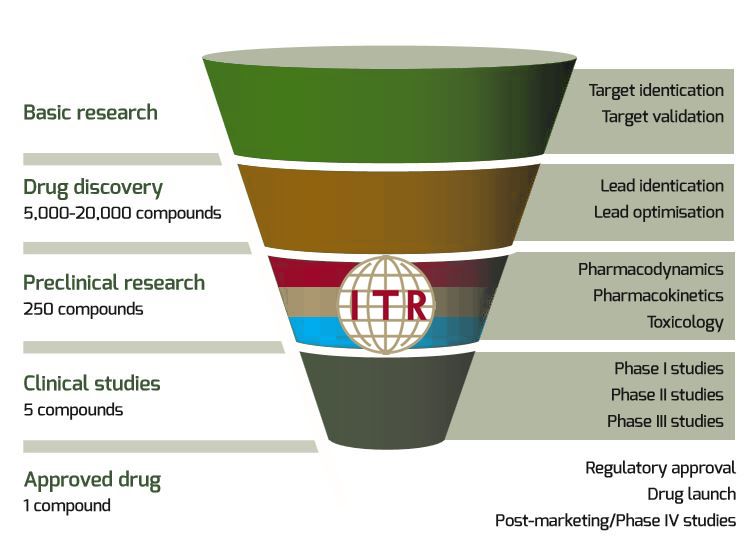
From Bench to Clinic - The Chronology of Preclinical Studies - ITR

Which Human Metabolites Have We MIST? Retrospective Analysis
Recomendado para você
-
 Lengkap Ada Video, Brain Test Level 411 Sang Ksatria Harus01 julho 2024
Lengkap Ada Video, Brain Test Level 411 Sang Ksatria Harus01 julho 2024 -
Water Sort Puzzle Color Sorting - Microsoft Apps01 julho 2024
-
 MicroRNA-411 and Its 5′-IsomiR Have Distinct Targets and Functions01 julho 2024
MicroRNA-411 and Its 5′-IsomiR Have Distinct Targets and Functions01 julho 2024 -
 The 411 on A1C: Normal A1C levels and 15 ways to lower high A1C01 julho 2024
The 411 on A1C: Normal A1C levels and 15 ways to lower high A1C01 julho 2024 -
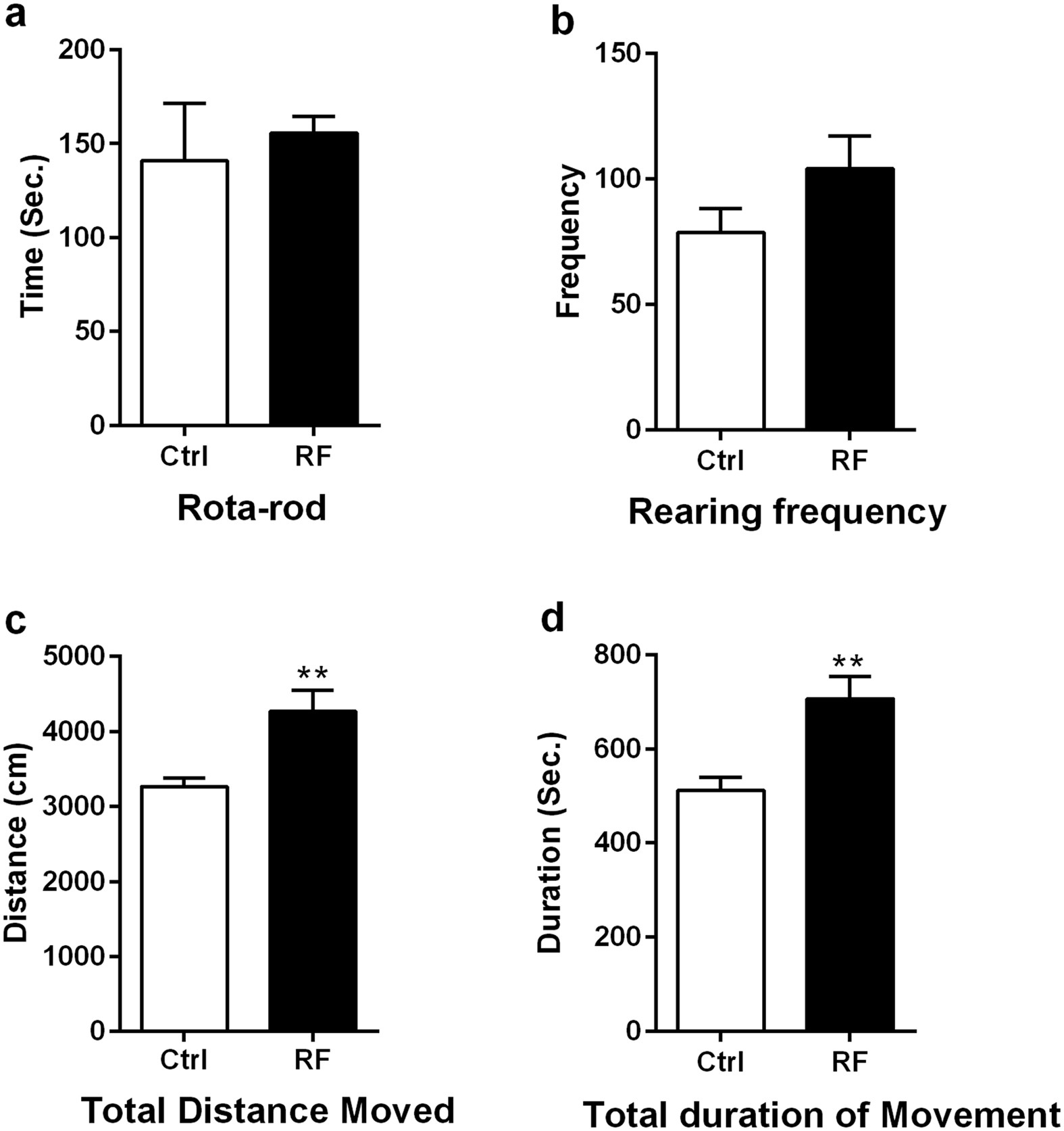 Long-term exposure to 835 MHz RF-EMF induces hyperactivity01 julho 2024
Long-term exposure to 835 MHz RF-EMF induces hyperactivity01 julho 2024 -
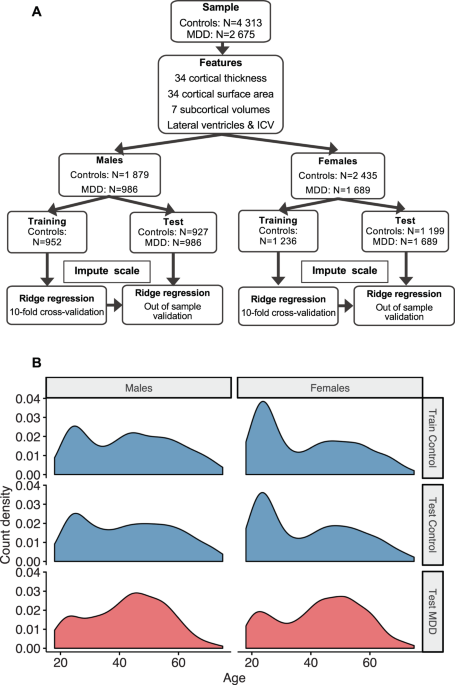 Brain aging in major depressive disorder: results from the ENIGMA01 julho 2024
Brain aging in major depressive disorder: results from the ENIGMA01 julho 2024 -
 411 Adrenal Insufficiency with Dr. Atil Kargi - The Curbsiders01 julho 2024
411 Adrenal Insufficiency with Dr. Atil Kargi - The Curbsiders01 julho 2024 -
 Pin on Health01 julho 2024
Pin on Health01 julho 2024 -
 Olfactory dysfunction in COVID-19: pathology and long-term01 julho 2024
Olfactory dysfunction in COVID-19: pathology and long-term01 julho 2024 -
 Wernicke's & Broca's aphasia Brain & Language LING 411/412/48901 julho 2024
Wernicke's & Broca's aphasia Brain & Language LING 411/412/48901 julho 2024
você pode gostar
-
 Tournament Of Kings (Las Vegas) Review01 julho 2024
Tournament Of Kings (Las Vegas) Review01 julho 2024 -
 BRASIL COLEÇÕES - Encontre o item que faltava para sua coleção !01 julho 2024
BRASIL COLEÇÕES - Encontre o item que faltava para sua coleção !01 julho 2024 -
 10 Great Light Novels That Never Got An Ending01 julho 2024
10 Great Light Novels That Never Got An Ending01 julho 2024 -
 Anime girl icon HD wallpapers01 julho 2024
Anime girl icon HD wallpapers01 julho 2024 -
 punch man: Overwatch 2 season 3 release date: Battle pass, One Punch Man, other key details - The Economic Times01 julho 2024
punch man: Overwatch 2 season 3 release date: Battle pass, One Punch Man, other key details - The Economic Times01 julho 2024 -
 Copa do Mundo – Jogar Agora01 julho 2024
Copa do Mundo – Jogar Agora01 julho 2024 -
 Vampirina Boneca de Pelucia desenho animado Mundo da pelúcia Pelúcias Game Land Brinquedos e Colecionáveis01 julho 2024
Vampirina Boneca de Pelucia desenho animado Mundo da pelúcia Pelúcias Game Land Brinquedos e Colecionáveis01 julho 2024 -
 Un loco transforma a Uruguay en un equipo fulminante en las01 julho 2024
Un loco transforma a Uruguay en un equipo fulminante en las01 julho 2024 -
 9 Elite Dangerous: Horizons Beginner Tips for Getting Started01 julho 2024
9 Elite Dangerous: Horizons Beginner Tips for Getting Started01 julho 2024 -
 Gorgonzola Quata Pç 3Kg01 julho 2024
Gorgonzola Quata Pç 3Kg01 julho 2024